5-Hydroxymethylthiazole is a kind of slightly yellow oil liquids, the Molecular Formula is C4H5NOS, Molecular weight is 115.15, and the CAS NO. is 38585-74-9. It is an important intermediate for Ritonavir, and a kind of anti-AIDS pharmaceuticals.
Since the discovery of 5-Hydroxymethylthiazole can be used for synthesis of second-generation anti-AIDS drugs ritonavir (Ritonavir), scientists have done many studies on the synthesis method of 5-Hydroxymethylthiazole. But how to prepare it, in this Article, I will introduce 3 new approaches:
Approach one
In a reaction flask IOOml 250ml of ethanol, 15g (0. Imol) 2_ chloro-5 - (hydroxymethyl) thiazole and 13. 5g (0. 20mol) zinc powder, the above mixture was heated to 50 C , then slowly add 150ml hydrochloric acid (2N), at a temperature of 70 C for 2 hours detected by TLC or HPLC, until the starting material disappeared, the reaction was stopped and the reaction solution was filtered, concentrated and neutralized with sodium hydroxide to PH of 10, and then extracted with ethyl acetate to give crude product, the final crude solvent was removed by distillation under reduced pressure, collecting 115 ~ 122 C (24mmHg) the distillate, 8. 5g 5 - (hydroxymethyl) thiazole. The determination of the purity% 99. 3% (HPLC), the yield was 74%.
Approach two
In a reaction flask IOOml 250ml of ethanol, 45g (0. 3mol) 2_ chloro-5 - (hydroxymethyl) thiazole and 33g (0. 5mol) zinc powder, and then added slowly 75ml hydrochloric acid (6N), the temperature of 60 C for 2 hours, by TLC or HPLC detection, until the starting material disappeared, the reaction was stopped and the reaction solution was filtered, concentrated and neutralized with sodium hydroxide to a PH of 10, and then extracted with ethyl acetate and to give the crude product, the final crude solvent was removed by distillation under reduced pressure, collecting 115 ~ 1220C (24mmHg) the distillate, 25g 5 - (hydroxymethyl) thiazole. The purity was determined 99. 5% (HPLC), the yield was 72%.
Approach three
2–Chloro-5-hydroxymethylthiazole hydrochloride(3.72 g 0.02 mole) was dissolved in methanol (30 mL) and charged into a Parr shaker. To this solution was charged sodium carbonate( 2.12 g 0.02 mole) and 10 palladium on carbon (0.9 g). The system was heated (60 C) under 50 psi (3.40 atm) of hydrogen gas and agitated for 18 hours. The reaction was monitored by TLC or GC and allowed to proceed for an additional 5 hours after completion. The reaction mixture was cooled and the contents filtered through a bed of diatomaceous earth. The filtrate was then concentrated under reduced pressure (38 C) and the residue was taken up in methyl t butyl ether (100 mL) and dried over sodium sulfate (10 g). The dried solution was filtered and concentrated under reduced pressure (38 C) to provide 5-hydroxymethylthiazole as a slightly colored oil Yield
Want to learn more information about 5-Hydroxymethylthiazole, you can access the guidechem.com. Guidechem.com is just a place for you to look for some chemicals. Guidechem provide the most convenient conditions for the international buyers and let these leads benefit all the business people.
2013年10月31日星期四
2013年9月22日星期日
A more efficient Synthesis of 5-Hydroxymethylthiazole
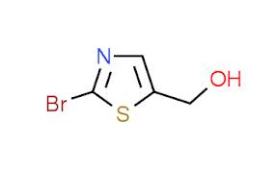 Since the discovery of 5-Hydroxymethylthiazole can be used for synthesis of second-generation anti-AIDS drugs ritonavir (Ritonavir), scientists have done many studies on the synthesis method of 5-Hydroxymethylthiazole.
Since the discovery of 5-Hydroxymethylthiazole can be used for synthesis of second-generation anti-AIDS drugs ritonavir (Ritonavir), scientists have done many studies on the synthesis method of 5-Hydroxymethylthiazole.
Typically, 5-Hydroxymethylthiazole synthesized mainly in two ways. The first one is 2 - chloro -5 - (chloromethyl) thiazole as the raw material by esterification, hydrolysis and catalytic hydrogenation dechlorination, after 3 steps Synthesis of 5-Hydroxymethylthiazole. The process requires the use of the esterification phase transfer catalyst, the reaction time is long, after-treatment troublesome, byproduct during the reaction are more, the need for active carbon, high cost.
The second is: 1-chloro-5 - (chloromethyl) thiazole dechlorination after hydrolysis and catalytic hydrogenation to give 5-Hydroxymethylthiazole(CAS NO: 38585-74-9) process, the process more hydrolysis byproducts, to active carbon, high cost; while catalytic hydrogenation dechlorination equipment investment, hydrogen explosive, requiring a higher security measures.
In this Article, I will introduce a new approach: 1 - chloro-5 - chloromethylthiazole as raw material through esterification, reduction and hydrolysis Synthesis of 5-Hydroxymethylthiazole process. Actually, we have done little change on this method, so that this method is more efficient.
Main raw materials and reagents
Materials: 2-chloro-5-chloromethyl thiazole, industrial grade; Zinc; Ethanol (95% mass fraction), ethyl acetate, acetic acid, acetate trihydrate Sodium, anhydrous sodium sulfate were of analytical grade.
Reagents: Perkin Elmer Clarus 500 Gas Chromatograph, Agi-lent LC-MSD1100 type liquid a mass spectrometer, VarianUnity INOUA-300-type superconducting NMR spectrometer, Thermo Fisher AVATAR 330 FT-IR. Perkin Eliner 2400-1I elemental analyzer.
Reagents: Perkin Elmer Clarus 500 Gas Chromatograph, Agi-lent LC-MSD1100 type liquid a mass spectrometer, VarianUnity INOUA-300-type superconducting NMR spectrometer, Thermo Fisher AVATAR 330 FT-IR. Perkin Eliner 2400-1I elemental analyzer.
Synthesis conditions:
Suitable esterification conditions: 2-chloro-5-chloromethyl-thiazole amount of 1mol, ethanol (boron = 80% to 90%) as a medium, material ratio: n (2-chloro-5-chloromethyl thiazole) : n (sodium acetate) = 1:2, refluxing for 5 ~ 6 h.
Reductive dechlorination process conditions: n (2 - chloro-5-chloromethyl thiazole): n (zinc) = 1:3, refluxing for 1 h.
Hydrolysis conditions: the base w (NaOH) in a 25% hydrolyzed, the reaction temperature is 70 ~ 75 ℃, reaction 2h. 5 - (hydroxymethyl) thiazole yield of 76.23%, w (5 a hydroxymethyl thiazole) ≥ 99% (GC, peak area normalization method). By 20I. After the reactor was scaled, 5-hydroxymethylthiazol yield increased to 81. 00%.
Process was improved, the esterification reaction does not require a phase transfer catalyst and ethyl acetate, solvent recovery and less consumption, the product simple treatment can be carried out after the next reaction; zinc / acetic acid reductive dechlorination method of operation simple, no catalyst is added, and the reaction time is short, easy to control; process improvement, the 5-Hydroxymethylthiazole increased yield from 54% to 76.23%.
2013年9月6日星期五
Two methods of preparation 5-Hydroxymethylthiazole
Chemical
and physical properties:
Product Name: 5-Hydroxymethylthiazole
English Synonyms: 5-Hydroxymethyl thiazole
CAS: 38585-74-9
EINECS Number: 414-780-9
Molecular formula: C4H5NOS
Molecular Weight: 115.15
Density: 1.339 g/cm3
Boiling Point: 248.5 ºC at 760 mmHg
Flash Point: 104.1 ºC
Risk Codes: 36/37/38-52/53-41-40
Safety Phrases: 26-36/37/39-61-22
Appearance: pale yellow oily liquid
Preparation
of 5- Hydroxymefhylthiazole:
Example
one:
2- Chloro -5- hydroxymethylthiazole (74.0 g 495 mmo)l was dissolved
in methanol (925 mL) and charged into a Parr shaker. To this solution was
charged sodium carbonate (26.76 g 252.5 mmol 0.51 eq) and 10 palladium on
carbon (11.1 g). The system was heated 60 C under 50 psi (3.40 atm) of hydrogen
gas and agitated for 8 hours. The reaction mixture can be vented periodically
to release the buildup of carbon dioxide gas. The shaker was then cooled and
the contents filtered through a bed of diatomaceous earth. The filtrate was
then concentrated under reduced pressure (38 C) and the residue was taken up in
methyl t butyl ether (600 mL) and dried over sodium sulfate (70 g). The dried
solution was filtered and concentrated under reduced pressure (38 C) to provide
5-hydroxymethylthiazole Yield 52.2 g, 91.6.
Example
two:
2–Chloro-5-hydroxymethylthiazole hydrochloride(3.72 g 0.02 mole) was
dissolved in methanol (30 mL) and charged into a Parr shaker. To this solution
was charged sodium carbonate( 2.12 g 0.02 mole) and 10 palladium on carbon (0.9
g). The system was heated (60 C) under 50 psi (3.40 atm) of hydrogen gas and
agitated for 18 hours. The reaction was monitored by TLC or GC and allowed to
proceed for an additional 5 hours after completion. The reaction mixture was
cooled and the contents filtered through a bed of diatomaceous earth. The
filtrate was then concentrated under reduced pressure (38 C) and the residue
was taken up in methyl t butyl ether (100 mL) and dried over sodium sulfate (10
g). The dried solution was filtered and concentrated under reduced pressure (38
C) to provide 5-hydroxymethylthiazole as a slightly colored oil Yield 2.05 g,
89.1.
Frankie is the freelance writer for e-commerce website in the
chemistry. Guidechem.com is just a place for you to look for some chemicals. Our guidechem
provide the most convenient conditions for the international buyers and let
these leads benefit all the business people. Guidechem chemical B2B network
provides information on china and global chemical market quotation and relative
chemical Information. Guidechem Chemical Network providing the most complete
information of the chemical industry.
订阅:
博文 (Atom)